Shaping the Future of Regenerative Medicine
BioTissue, a subsidiary of TissueTech, is the pioneer and leader in regenerative medicine for ocular surface disease and injury, as well as ocular hygiene solutions for eye care professionals. Established in 1997, BioTissue serves an unmet need for better surgical and therapeutic alternatives for helping eye care professionals manage ocular surface conditions, such as keratitis, recurrent corneal erosions, conjunctivochalasis (CCh) also known as mechanical dry eye, pterygium and severe dry eye.
Eye care professionals understand that good medicine is based on good science. BioTissue prides itself on developing products that meet the highest clinical standards, are based on quality research and scientific data, and provide innovative solutions for the management of a range of ocular surface and lid margin diseases. BioTissue adheres to the FDA tissue and medical device guidelines.
Promote Regenerative Healing, Backed by Research
Our extensive research allows us to preserve and deliver the most functional allograft for your needs.
500K
TRANSPLANTS
360
CLINICAL PUBLICATIONS
30Y
RESEARCH & DEVELOPMENT
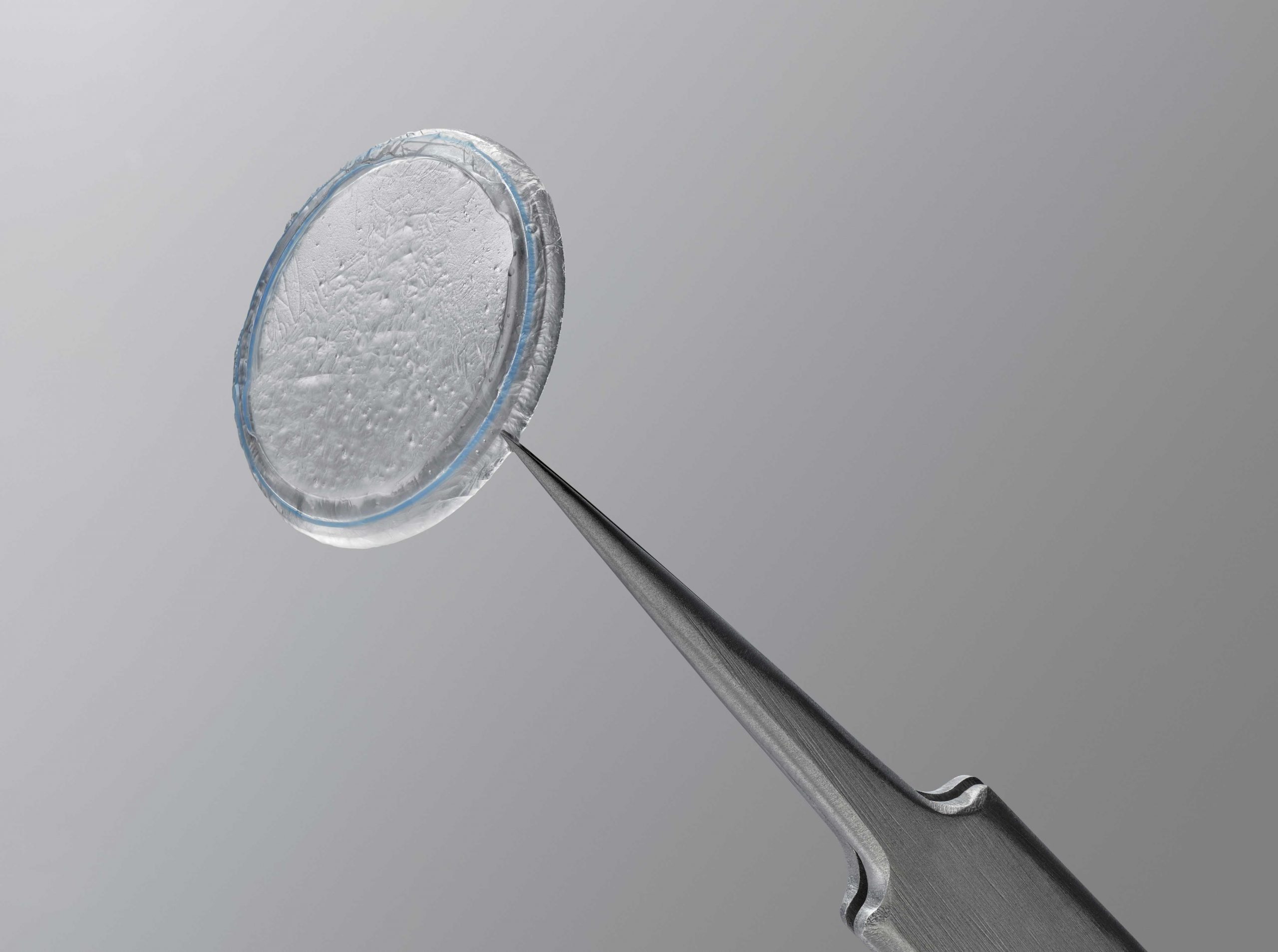

PROKERA® biologic corneal bandage devices are used by eye doctors around the world to heal and treat ocular conditions such as keratitis, moderate to severe dry eye disease, recurrent corneal erosions, filamentary keratitis, persistent epithelial defects, neurotrophic corneas, herpetic ulcers, and many other ocular surface diseases such as chemical burns and Stevens-Johnson syndrome.

![]()
AmnioGraft is a biologic ocular transplantation graft used by eye surgeons around the world as an adjunct therapy to treat ocular surface indications such as corneal ulcers, pterygium, mechanical dry eye (also known as conjunctivochalasis), excision of tumors, chemical burns, and Stevens-Johnson Syndrome. AmnioGraft serves as a tissue replacement by delivering the unique therapeutic actions of cryopreserved amniotic membrane.

![]()
AmnioGuard is an umbilical cord graft used by ophthalmic surgeons for a number of indications including to cover the tube shunt of a glaucoma drainage device. Due to its high tensile strength and optimally designed thickness, AmnioGuard can reduce surgical times because it does not require rehydration, is easy to handle and suture, and can be used for corneal, conjunctival, sclera, fornix, socket and eyelids where thickness and high tensile strength are desired.
See What Others Are Saying About Our Products
See What Others Are Saying About Our Products
Paul Karpecki, OD
“Cryopreserved maintains a lot of the essential components that are necessary for healing. It also has the research and there is lots of scientific sources that show nerve regeneration for example, which we have not seen with any other form of amniotic membrane.”
Neel Desai, MD
“We’ve all had the paradoxical patient that seems to have dry eye and typical ocular surface disease, but they simply don’t respond to all of the typical conventional therapies…there is a missing x-factor. And that x-factor is this Mechanical Dry Eye concept, or conjunctivochalasis.”
Marguerite McDonald, MD
“I put on a second one and took it off a week later, she was 20/20 on both eyes, we canceled the surgery and I became a believer. That was my Wow Moment”
DISCLAIMER
BioTissue, Inc. will use reasonable efforts to include accurate and up-to-date information on this Website but makes no warranties or representations of any kind as to its accuracy, currency, or completeness.
The contents of this web site are protected by applicable copyright laws. No permission is granted to copy, distribute, modify, post or frame any text, graphics, video, audio, software code, or user interface design or logos.
The information contained on this website is for general information and educational purposes only, and is not intended to diagnose, treat, or cure any medical conditions. Please consult with your Physician for medical advice.
BioTissue, Inc. and its affiliates furnish this allograft product without any express or implied warranties. All statements or descriptions are informational only and are not to be interpreted or implied as a warranty of the allograft product. BioTissue, Inc. and its affiliates make no guarantee regarding the biological characteristics of this product. The end user shall be held responsible for determining the appropriate application and usage of this product.
